Abstract
Background: The optimal approach for patients with smoldering multiple myeloma (SMM) at a high risk of progression remains an area of active investigation. Two phase 3 trials have demonstrated reduced risk of progression to active MM when patients with high risk SMM are treated with lenalidomide with or without dexamethasone compared with observation alone, with one study also showing improved overall survival. In addition to reducing the risk of progression, intense therapy applied at this precursor phase can potentially eradicate the malignant clone and lead to long term remissions or even cure. The ASCENT trial (NCT03289299) was designed to test this hypothesis.
Patients and methods: Patients with a diagnosis of SMM based on the IMWG revised diagnostic criteria, were enrolled if they met criteria for high risk by IMWG 20/2/20 staging system or a total score ≥9 on the IMWG scoring system. Patients required adequate hematological and organ function and should have no evidence of amyloidosis. The primary endpoint for the study was the rate of stringent complete responses (sCR) at end of maintenance, and secondary objectives included MRD negativity rate (10-5) and progression free survival (PFS). Treatment consisted of 3 phases: induction (cycles 1-6), consolidation (cycles 7-12), and maintenance (cycles 13-24), with treatment limited to 24 cycles, each cycle being 28 days. Carfilzomib was given at 56 mg/m2 IV days 1, 8, 15 for cycles 1-12; lenalidomide, at 25 mg PO daily days 1-21 for cycles 1-12 and then reduced to 10 mg; daratumumab, at 16 mg/kg IV weekly for cycles 1-2, every other week cycles 3-6 and then once every cycle during induction and every other cycle during maintenance; and dexamethasone, weekly at 40 mg for cycles 1-6 and 20 mg for cycles 7-12 and then discontinued. Disease assessments were performed by IMWG response criteria every cycle and MRD was assessed by Euroflow at end of induction, consolidation, and maintenance.
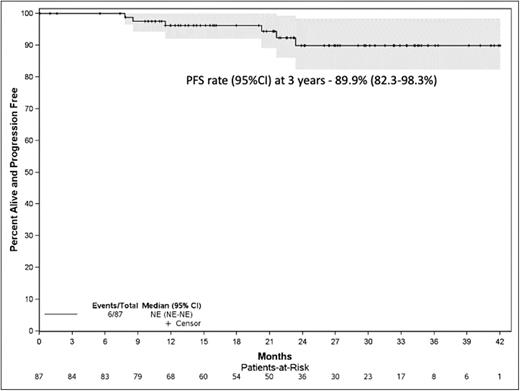
Results: Eighty-seven patients were enrolled on the trial between July 2018 and Nov 2021 from 10 US centers; median age was 64, 50.6% were male. Median follow up on the study was 25.8 (95% CI, 21.3-29.1) months; 38 patients remain on treatment. Forty-one (47%) patients have completed all 24 cycles; median number of cycles administered were 21, ranging 1 to 24. Ten patients went off study prior to completion of 24 cycles; due to withdrawal by subject (3), adverse events (2), physician decision (3), progression (1), and death (1). The best overall response rate was 97%, with 37% sCR, 26% CR, 29% VGPR, 2% PR, 1% SD, and 2% NE. Seventy-three (84%) patients became marrow MRDneg (53 [61%] also in CR - IMWG MRDneg); at a median time to MRD negativity of 6.6 months; 53 at end of induction, 16 at end of consolidation and 4 at end of maintenance. Three patients have progressed, median PFS for the cohort has not been reached; PFS rate (95%CI) at 3 years was 89.9% (82.3-98.3%). Any grade toxicity, possibly related to therapy, was observed in 83 (97%) patients. Any grade 3 or higher hematological toxicity in 13 (15%) patients and non-hematological toxicity in 44 (51%) patients. The median dose per cycle of lenalidomide was 250 mg, dexamethasone was 60 mg, 204 mg for carfilzomib, and 1,600 mg for daratumumab. Dose reductions were required for carfilzomib in 11 patients, lenalidomide in 9 patients and dexamethasone in 14 patients.
Conclusions: The combination of daratumumab, carfilzomib, lenalidomide and dexamethasone given for a fixed duration of 2 years was associated with high response rates and deep responses including high rates of MRD negativity. The regimen was well tolerated, with few grade 3 or 4 toxicity. The responses appear durable as indicated by the sustained MRD negativity as well as the 3-year PFS rate of 90%. With nearly half of the patients still receiving therapy, the depth of response is expected to improve.
Disclosures
Kumar:AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Research Funding; MedImmune/Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee. Alsina:BMS: Research Funding; BMS, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Badros:GSK, BMS: Research Funding; Amgen: Consultancy. Abonour:Janssen: Honoraria, Research Funding, Speakers Bureau; GSK: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Amgen: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Prothena: Honoraria. Dhakal:Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding, Speakers Bureau; Karyopharm Therapeutics: Honoraria, Speakers Bureau; BMS: Honoraria, Research Funding; Natera: Consultancy; Arcellx: Research Funding; Carsgen: Research Funding; Cartesian: Research Funding; Fate: Research Funding; Takeda: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees. Bhutani:MedImmune: Research Funding; Janssen: Consultancy, Research Funding; Legend BioTech: Research Funding; Amgen: Research Funding; Celularity: Research Funding; Adaptive Biotechnologies: Research Funding; BMS: Research Funding; Celgene: Research Funding; Bluebird Bio: Research Funding; Millenium: Research Funding; Avalo therapeutics: Research Funding; C4 Therapeutics: Research Funding; Takeda: Research Funding; Sanofi: Consultancy. Jakubowiak:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Durie:Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria.
OffLabel Disclosure:
Daratumumab, Carfilzomib, Lenalidomide And Dexamethasone For Smoldering Multiple Myeloma
Author notes
Asterisk with author names denotes non-ASH members.